Functional dynamics of biomolecules
Staff:
Michel Sliwa
Marten Vos
Post-doc:
Amira Gharbi
Research topics:
Protein functioning relies on their capacity to efficiently adopt distinct configurations. Our research concerns the internal dynamics associated with these specific changes, in particular in proteins involved in catalysis, signaling and engineered photoswitches. The fastest and most specific changes take place on the timescale of the internal protein vibrations. Therefore we employ and develop femtosecond spectroscopic techniques to probe these motions in real time. Our efforts to get detailed insight in the dynamics on a molecular level include combining experimental studies and molecular dynamics simulations.
These studies rely on triggering protein dynamics using short visible light pulses. Therefore we investigate systems harboring chromophores, including flavin, heme, Fe-S center, retinal, carotenoid and chromophores constituted by post-translationally modified amino-acids.
Present projects include:
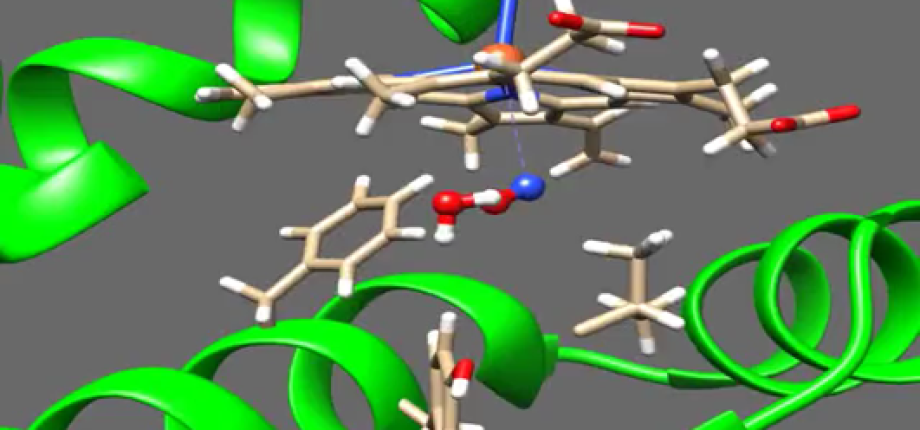
Photocatalytic mechanism of flavoenzyme Fatty Acid Photodecarboxylase (FAP).
Photodissociation of the charge transfer complex between the flavoprotein MSOX and its MTA inhibitor and its possible photoswitching applications (ANR PhotoCT, 2024-2027).
Signaling mechanism of Orange Carotenoid Protein (OCP) (ANR DynOCP)
Photoswitching of the retinal protein Archearhodopsin-3 (ANR UltrAerchea)
Mechanism of reversibly switchable fluorescence proteins
Much work is developed along with other themes in our lab, in particular "Adaptive molecular mechanisms in microbial systems" and "Coherent spectroscopy and coherent control in biological systems", and in addition with a number of external collaborations.
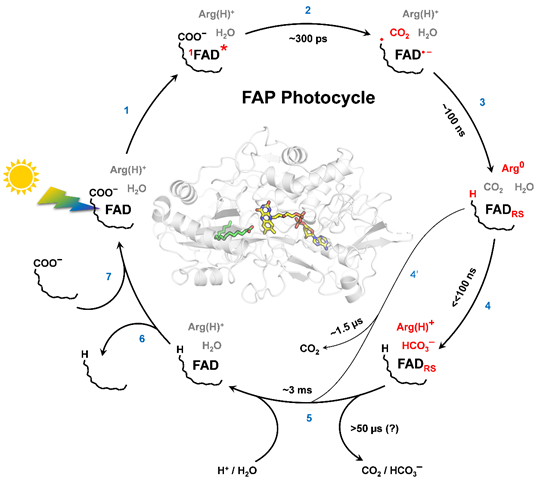
Photocycle of Fatty Acid Photodecarboxylase (FAP). See Sorigué, D. et al, (2021) Science 372, eabd5687
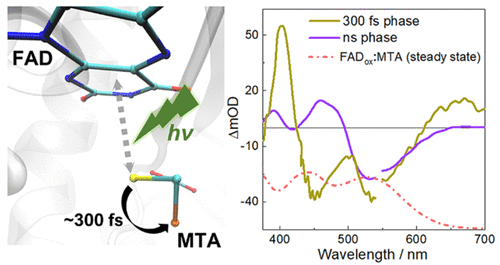
Photoswitching in the MSOX-MTA complex. See Zhuang, B. & Vos, M.H. (2022) J. Am. Chem. Soc. 144, 11569-11573
Selected recent publications:
Aleksandrov, A., Bonvalet, A., Müller, P., Sorigué, D., Beisson, F., Antonucci, L., Solinas, X., Joffre, M. & Vos, M.H. (2024) Catalytic Mechanism of Fatty Acid Photodecarboxylase: on the Detection and Stability of the Initial Carbonyloxy Radical Intermediate, Ang. Chem. Int. Ed. 63, e202401376
Zhuang, B., Liebl, U. & Vos, M.H. (2022) Flavoprotein photochemistry: fundamental processes and photocatalytic perspectives, J. Phys. Chem. B 126, 3199-3207



