Coherent spectroscopy in biomolecules
We use and develop spectroscopic techniques using femtosecond lasers to understand how structural changes in proteins contribute to the unfolding of a biological reaction.
The combination of ultra-short infrared pulses and advanced femtosecond technologies, such as coherent control and coherent spectroscopy, constitutes a highly powerful tool for studying the femtosecond dynamics of biological systems like hemoproteins. The current projects are as follows:
- Ultrafast vibrational spectroscopy enables the study of structural changes accompanying ligand transfer within molecules such as hemoproteins, with femtosecond temporal resolution.
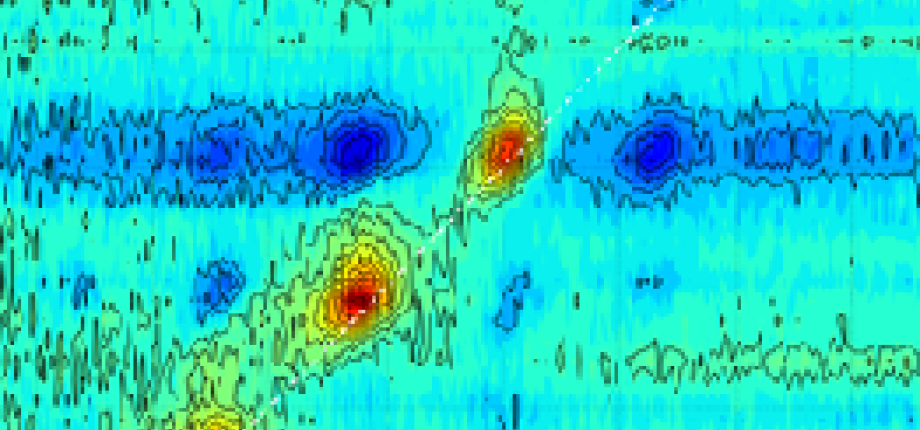
- Mid-infrared multidimensional spectroscopy (2DIR), a technique we initiated in 1996, has since been extensively developed and refined in many laboratories. In the infrared domain, it provides access to couplings between vibrational modes with sub-picosecond resolution.
- Vibrational climbing is a technique for controlling the vibrational motion of a chemical bond. It relies on the use of an infrared pulse that selectively excites a specific vibration while accounting for the anharmonicity of the vibration through frequency chirping.
- AD-ASOPS (Arbitrary Detuning-ASynchronous Optical Sampling) is a technique we developed and patented. It uses two asynchronous laser sources to measure the response of a system with sub-picosecond temporal resolution over time scales ranging from milliseconds to femtoseconds.




