Cell signaling nanoimaging

Oxidative signaling
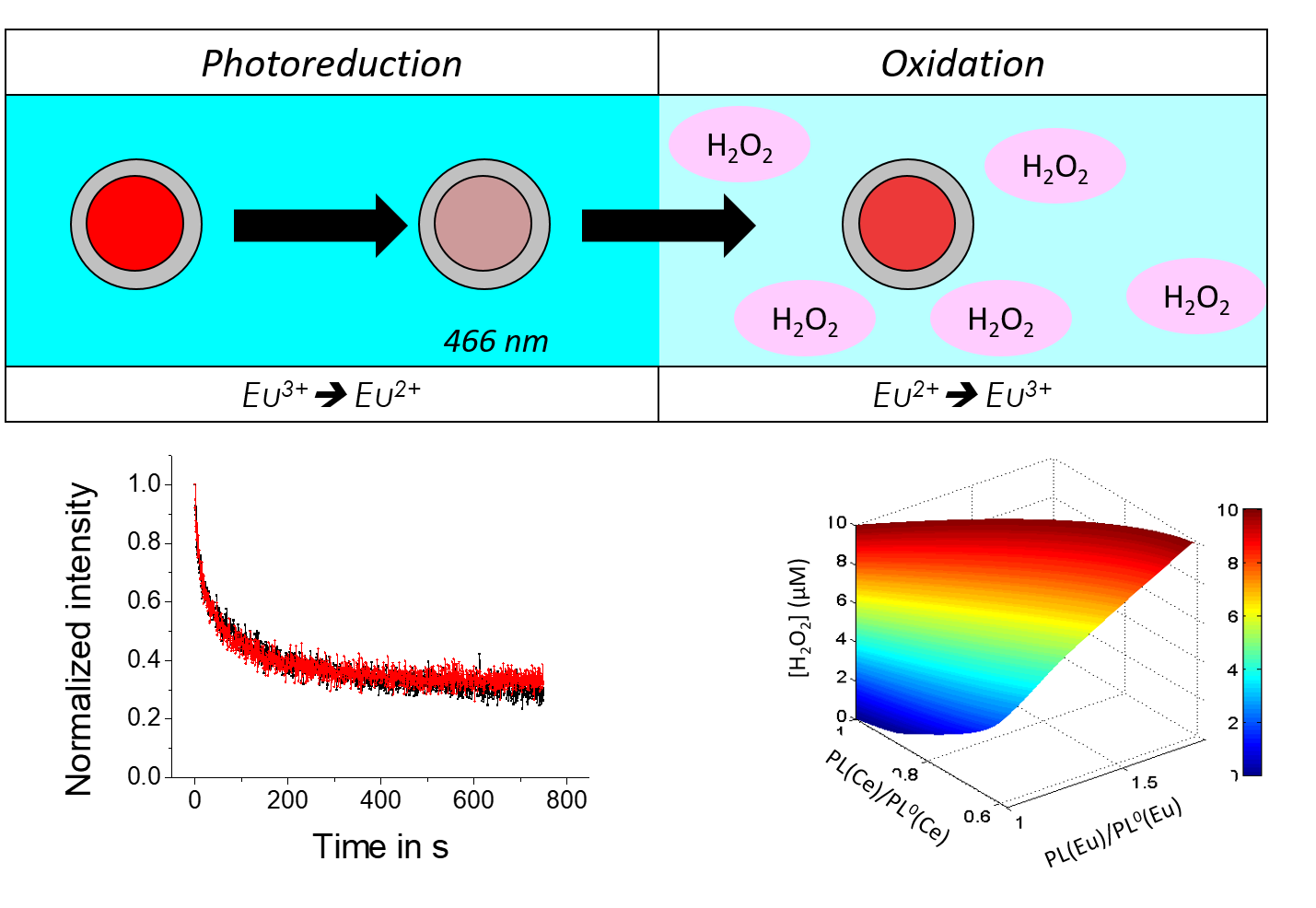
Reactive Oxygen Species (or ROS), such as H2O2, play a major in numerous patho-physiolgical process, notably in the triggering and evolution of complex patholgoies, such as some cancers or (auto)-inflammation related diseases. Their quantitative monitoring in cells and tissues is thus essential for their deciphering and could be a major asset in the perspective of designing future treatments. However, current methods of ROS detection or imaging often lack the quantitativity and/or the dynamics to address this issue and accurately reveal signaling mechanisms. We are thus developing the use of lanthanide-based nanoparticle as nanosensors for quantiative ROS imaging at high spatial and temporal resolution. YVO4:Eu nanoparticles are indeed luminescent through the presence of Eu3+ doping ions, whose redox state can be modulated optically or chemically. Through single nanoparticle imaging, the oxidative properties of their environment can thus be monitored quantitatively (Figure 1).
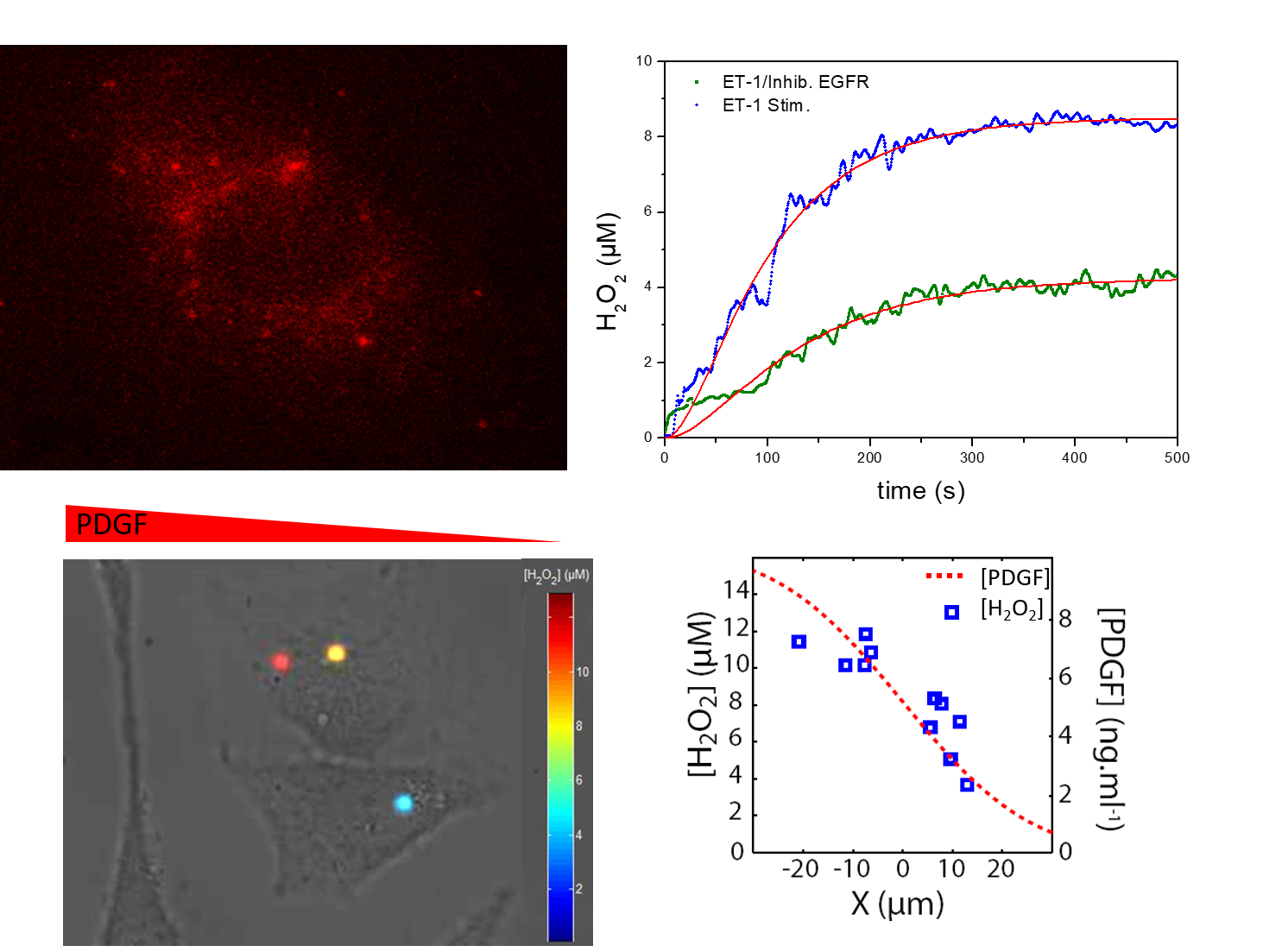
The use of redox sensititve in cells allows the measurement of intracellular ROS concentration in response to various stimuli. We notably study the oxidative signaling in vascular and kidney cell to reveal the molecular mechanisms controlling their pathos-physiological behavior, including contraction, proliferation and migration. We notably identify regulation mechanisms (involving EGF, PDGF, ET-1,.. pathways) of ROS homeostasis and their organization and dynamics at sub-cellular scales in normal or pathological, vascular, kidney,... cells in (Figure 2)
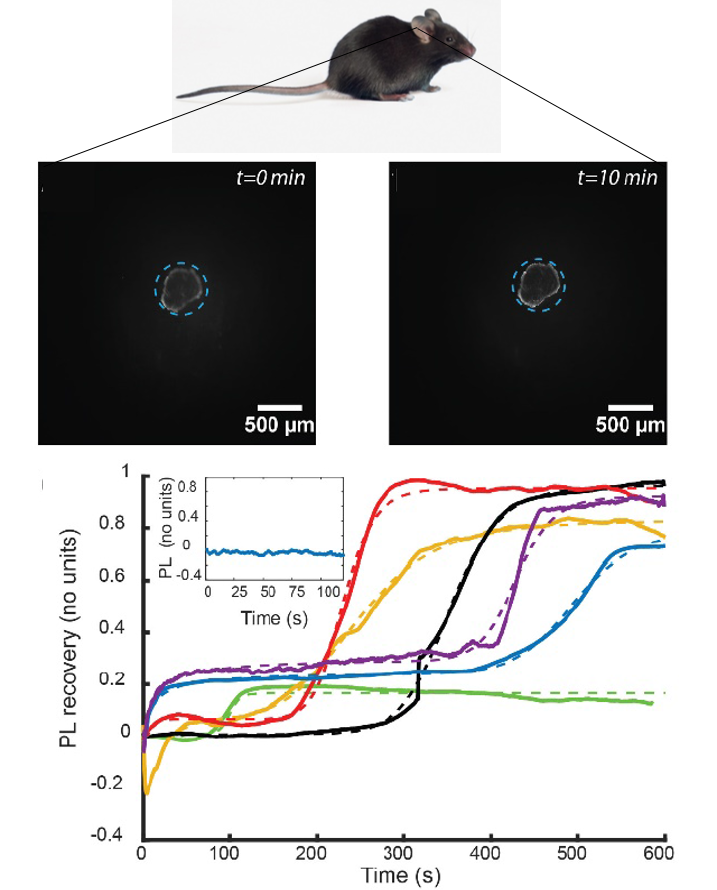
Based on the nanoparticle luminescence properties, we are furthermore developing methodologies for ROS probing at larger scales, either on-chip or in vivo (FIgure 3) in pathological models. The objective of this work is thus the monitoring of pathological, e.g. inflammation-related, transitions at the molecular scale, in complex, physiologically relevant, systems.
Membrane receptor nano-dynamics
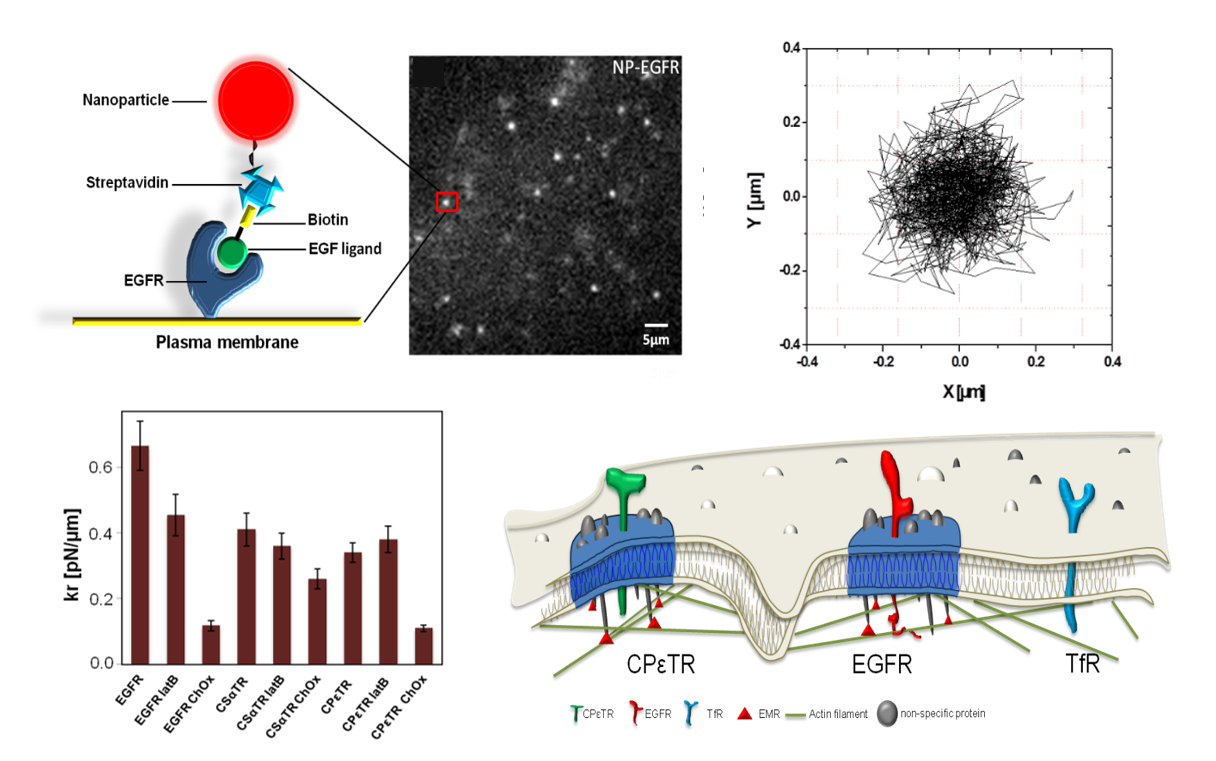
The cell response to various stimuli rely on the spatial organization of the involved signaling pathways, notably the activation of membrane receptors, which may be regulated by their dynamics and organization. In order to probe these properties and their functional role, we develop the tracking of single membrane proteins through luminescent nanoparticles (YVO4:Eu) imaging. This method provides minute-long trajectories of single receptors at high spatial and temporal resolution (typically 30 nm/20 ms) in living cells (Figure 4), whose analysis through advanced numerical methods may provide a quantitative characterization of their mobility and of the energy landscape regulating their nano-organization. Based on this approach, we notably classified the type of confinement of different receptors (EGFR, toxin receptors,...) and identified their molecular regulators (Figure 4). We furthermore investigate the effect of this membrane organization impairment in receptor activation (EGFR) and subsequent cell response, notably in the context of pathological kidney function regulation. These studies contribute at identifying quantitatively critical events at the molecular scale, which may then constitute key target in the regulation of cell and organ functions.
References
Confinement energy landscape classification reveals membrane receptor nano-organization mechanisms
In vivo ROS production during inflammation revealed by lanthanide-based nanoparticle imaging
Fast quantitative ROS detection based on single multi-color lanthanide nanoparticle imaging Dual-color imaging of lanthanide-based single nanoparticles enables fast quantitative ROS detection and reveals endothelin-1 signaling pathway kinetics in living cells
Mapping the intracellular concentration of reactive oxygen species.
Regulation of the ROS Response Dynamics and Organization to PDGF Motile Stimuli Revealed by Single Nanoparticle Imaging. Bouzigues CI, Nguyên TL, Ramodiharilafy R, Claeson A, Tharaux PL, Alexandrou A Chem. Biol. 21, 647 (2014)
Single Eu-doped nanoparticles measure temporal pattern of reactive oxygen species production inside cells.



