Microfluidics and organs-on-chips
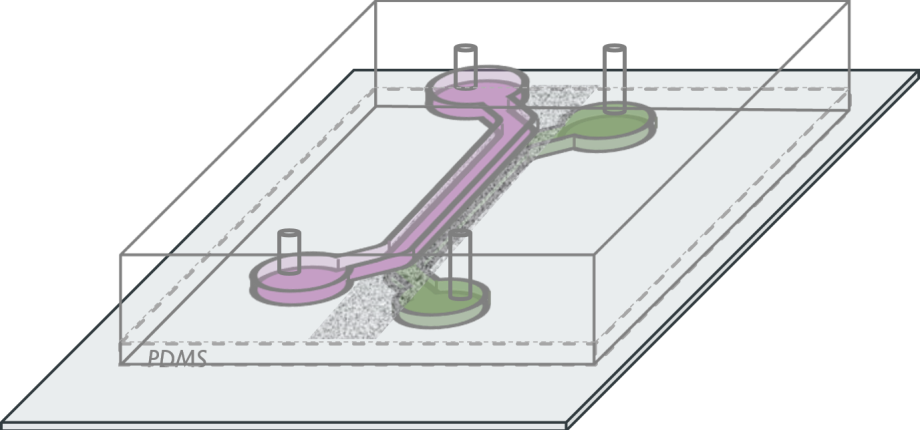
The deciphering of complex patho-physiological processes, such as the triggering of multi-factorial diseases, relies on the possibility to perform accurate measurement in systems capturing essential features. In this context, we are developing methodologies to produce opto-fluidic systems, in which both physiological environment and stimuli can be mimicked, and in which efficient imaging methods can be implemented to quantify cell and/or tissue at different scales.
Probing cell behavior through microfluidics and imaging
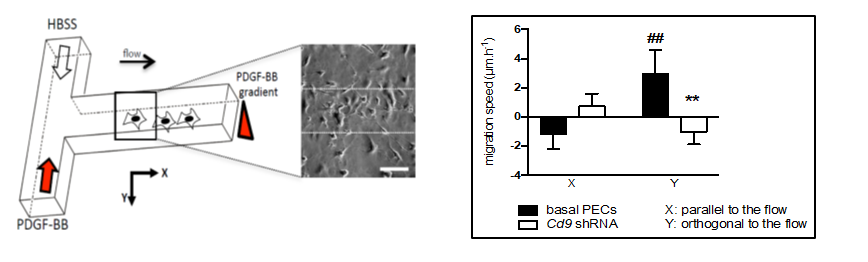
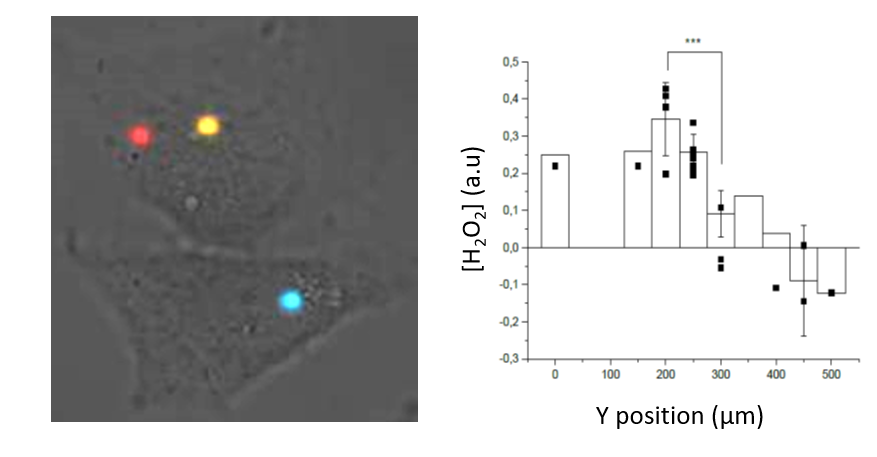
Based on this approach, we probe the responses at the cell and molecular scale, i.e. single cell motility, ROS production, membrane receptor organization, to organized stimuli, such as chemotactic gradients or mechanical stress, in different contexts (kidney PECs, cervical tumoral cells,...). This contributes to the understanding of the regulation mechanisms of normal or pathological migration (Figure 1).

Imaging in organs-on-chips
The triggering and evolution of complex pathologies often involve the interplay of numerous events occurring at the molecular, cell and tissue scale. In this context, usual in vitro cell approaches may be insufficient to capture essential features of the biological system, while accurate quantitative measurement methods may be lacking in in vivo models. We are thus developing opto-fluidic devices, or imageable organs-on-chips, in which multi-cells systems mimick the organization and function of actual organs, while remaining accessible to sensitive imaging methods and controlled chemical or mechanical stimuli. We are notably using these methods for the realization of a functional glomerulus (the filtration unit of the kidney)-on-chip, in which features encountered in kidney pathologies, such as some glomerulonephrities, can be elicited, imaged and quantitatively characterized.
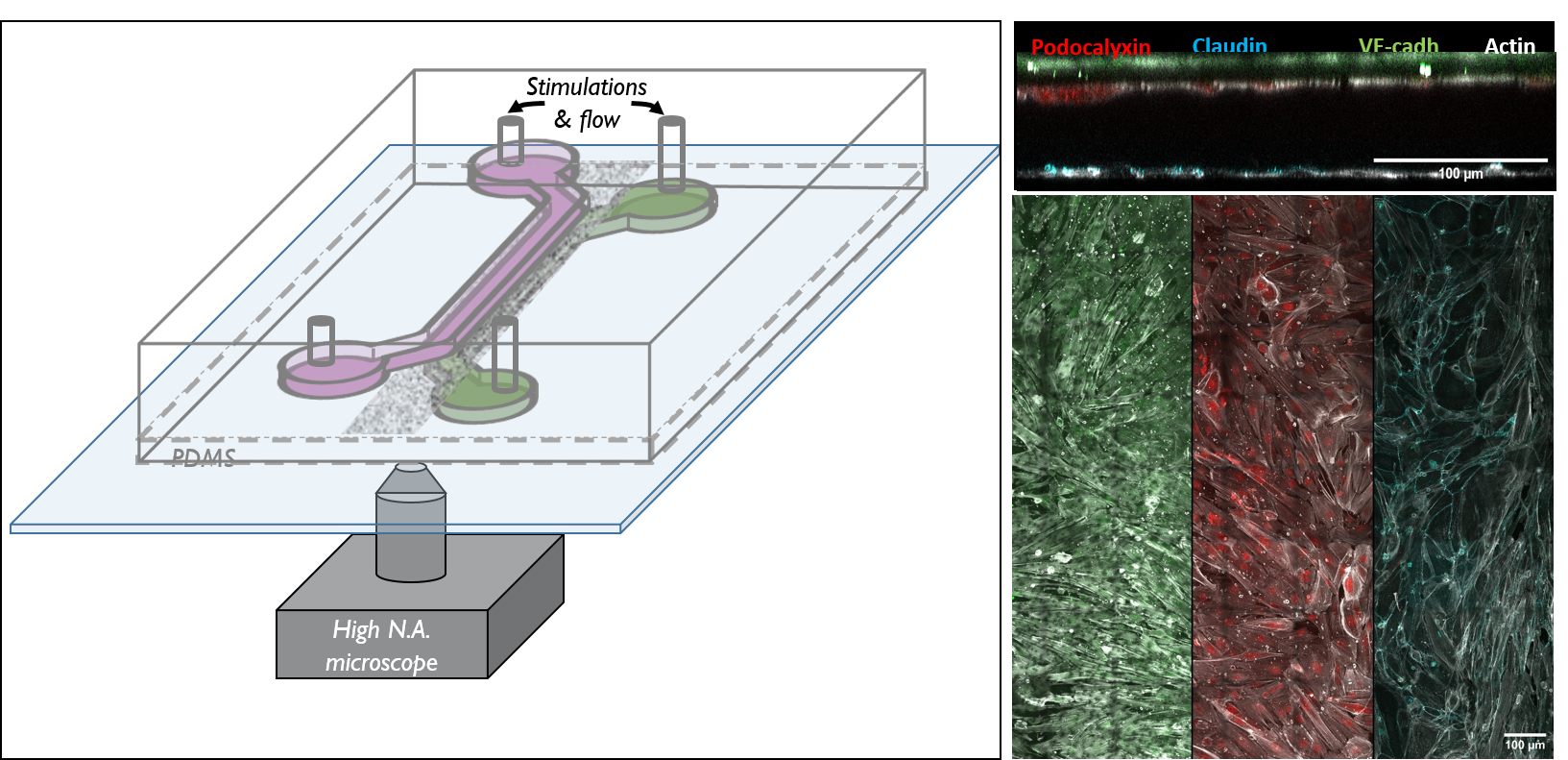
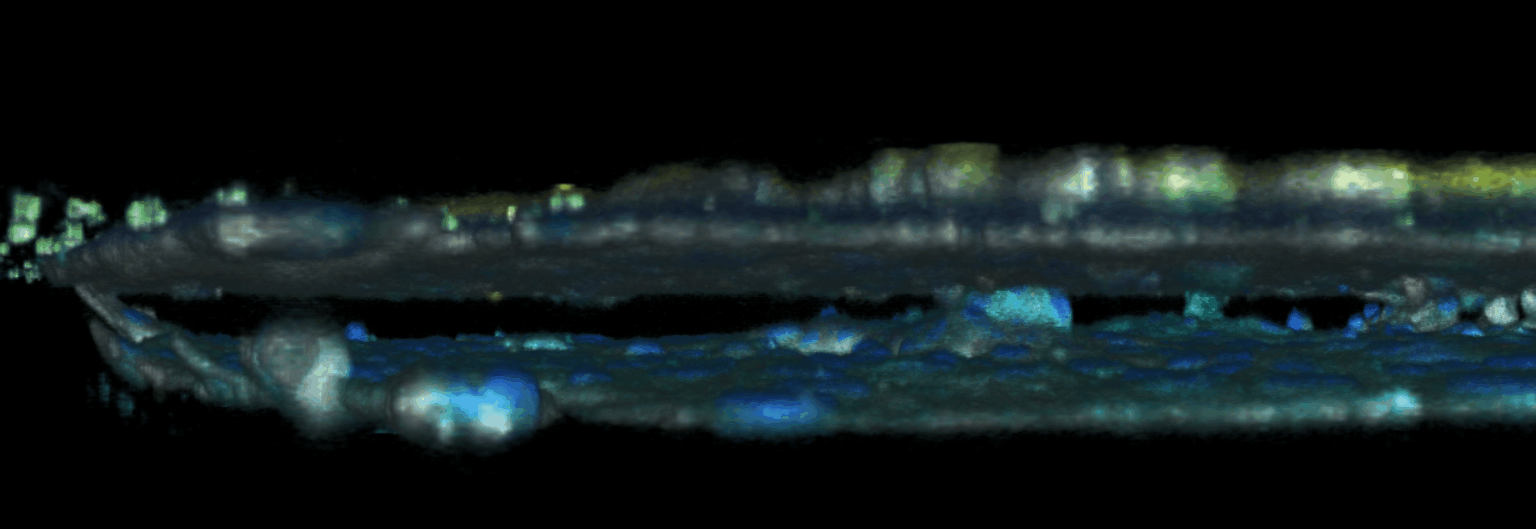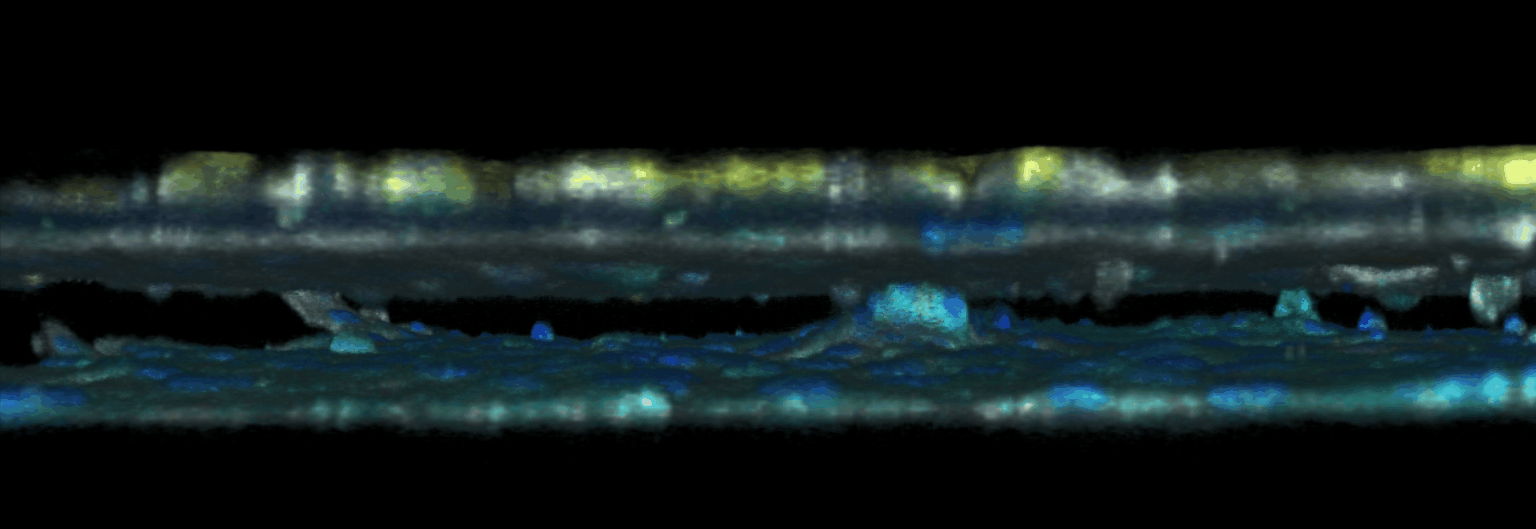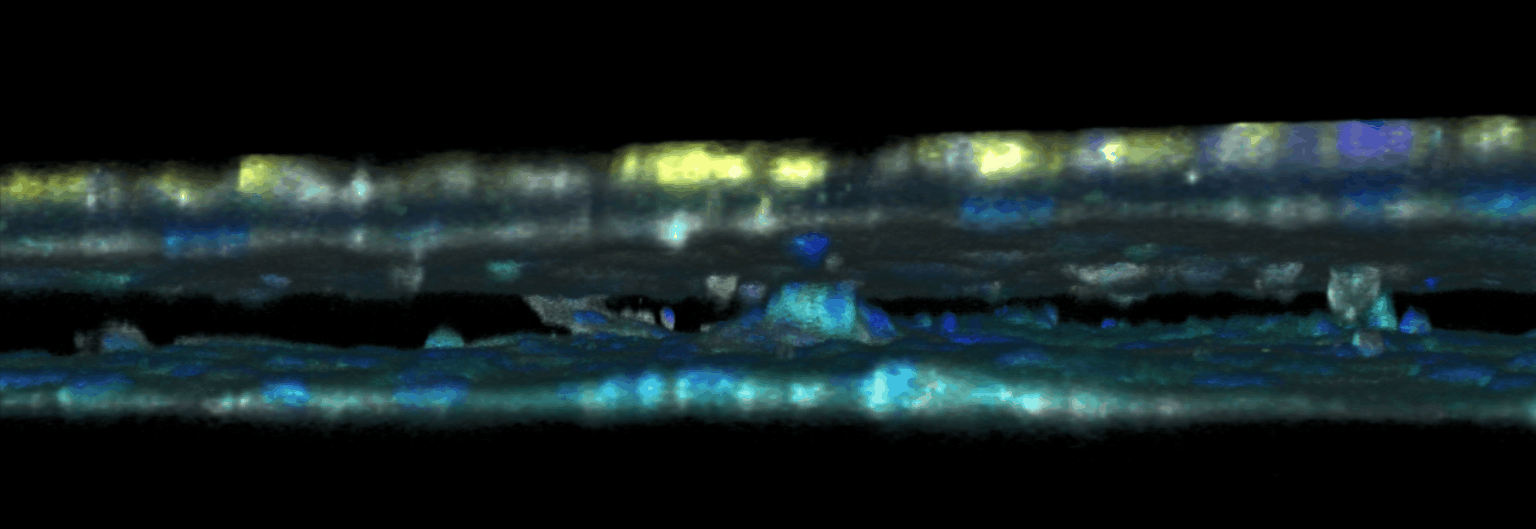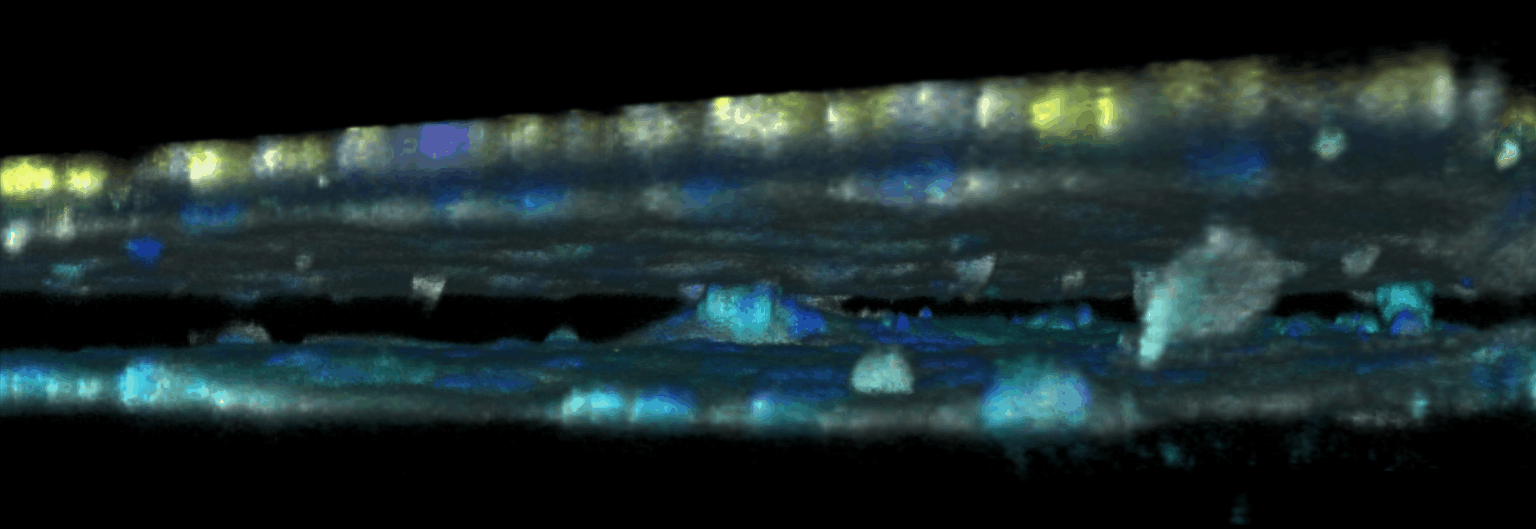
This approach combining biomimetic system design and quantitative imaging is the basis to further quantitative phenotypic screening of mulitple stimuli, in order to identify critical mechanism of pathological transitions and possible strategies of control.



