The mechanism, targeting, and evolution of the alternative thymidylate synthase ThyX
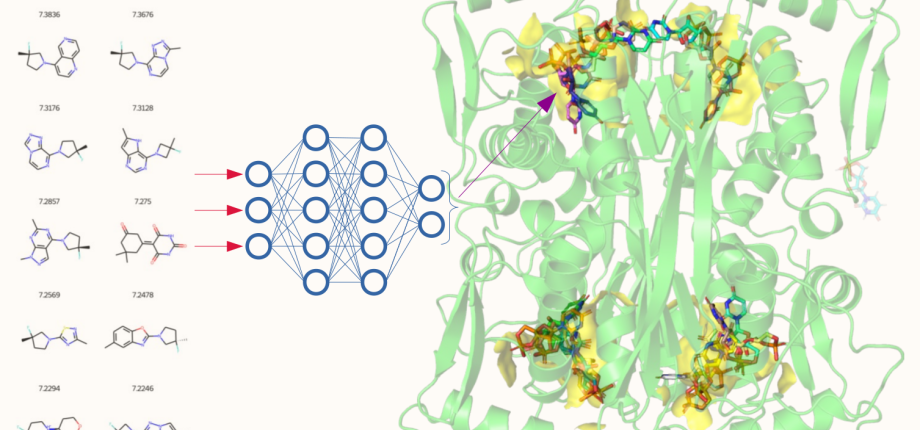
Much recent work has been devoted to molecular biological and biochemical studies to unravel the reaction mechanism and inhibition of alternative thymidylate synthase ThyX, discovered in the laboratory. ThyX proteins are essential for de novo synthesis of the letter “T” (thymidylate) in DNA in many prokaryotes. Our work has shed new light on the evolution of prokaryotic genomes, since the low catalytic activity of ThyX limits DNA replication and consequently is found in prokaryotic species with small genomes, whereas organisms with more complex genomes use thymidylate synthase ThyA, the enzyme present also in humans. As ThyX is present in many human pathogens such as Helicobacter pylori or Mycobacterium tuberculosis but absent in human cells, this opens the way to the development of novel antimicrobial strategies by identifying new inhibitors of this enzyme. In collaboration with Team 2 (LOB), several complementary techniques have been applied to research on ThyX. In particular, we have observed unusually high flexibility of the active site of ThyX. We are currently interested in evolutionary histories of the thymidylate synthase families. We have proposed how complex substrate interactions modulate oxygen reactivity of ThyX proteins. We are also analyzing folate metabolic and related pathways in the human pathogenic bacterium H. pylori. Finally, we have obtained proof–of-concept using animal models of infection for the anti-microbial activity of 1,4-naphthoquinones that we patented as efficient and specific inhibitors of the alternative thymidylate synthase X (Figure 1). Our primary goal is to render our inhibitor series for eventual preclinical development through collaborative efforts. This will require synthesis of new lead derivatives to optimize anti-microbial activity of already identified compounds.

Figure 1: 3D structure of ThyX (left); Representation of the C8-C1 inhibitor fitted into ThyX catalytic site (middle) (adapted from Basta et al (2012); Antibiotic susceptibility testing of ThyX inhibitors (right).



