Functions and diversity of circular RNAs in archaea
Thematic members
Hubert Becker, Professeur des universités, Sorbonne Université (resp.) (ORCID ID)
Maria Imezar, doctorante PhD
Manon AGOSTINI, doctorante PhD
Caroline Lhermitte-Stead, Ingénieur d’étude, INSERM
Alexey ALEKSANDROV, CR, CNRS
Hannu Myllykallio, DR, CNRS
Research Topic
Archaea are fascinating organisms with many bacterial features, but with information-processing systems homologous to eukaryotic counterparts (e.g. replication, transcription, translation, etc.). Archaeal research has led to many change-of-concepts mechanistic discoveries and their global environmental impact is currently being unraveled. We are interested in the different aspects of archaeal molecular biology, and especially in circular RNAs that are covalently closed molecules, lacking 5’- and 3’ free ends. As circular RNAs are no longer regarded as aberrant splicing byproducts, it is essential to uncover their functional potential and to elucidate the mechanisms of their formation.
Circular box C/D RNAs P. abyssi and the new Pab1020 RNA ligase :
• Over the past 10 years, several archaeal RNA transcriptomes from next-generation sequencing technologies have revealed a genome-wide presence of circular RNA. Our transcriptomic study in P. abyssi cells, an hyperthermophilic archaea, identified 133 circRNAs (Becker et al, Biochimie 2019). Interestingly, there was a high overrepresentation of 38 Box C/D RNAs that direct the 2’-O-methylation of ribosomal RNA (Figure 1). Our team is interested in determining the function of the circular form of Box C/D RNAs, guides site-specific for rRNA modification (2’O-methylayion) by molecular biology and biochemistry approaches. The precise role of the circular form of Box C/D RNAs in this methylation process is not yet understood.
Figure 1 A. Circular RNA-seq of P. abyssi, with the number of reads supporting circRNAs B. Schematic structure of archaeal Box C/D-RNA, (~55 nucleotides)
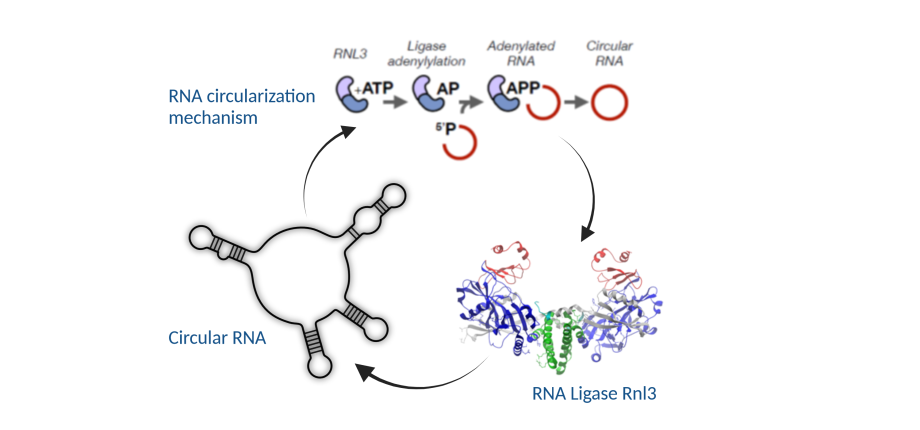
• We have identified the Pab1020 RNA ligase, a member of the Rnl3 family (Becker et al, RNA Biology 2017), which performs the RNA circularization reaction in P. abyssi, and we are working now on solving the mechanism and the RNA-Protein interaction mode.
• We determined the protein network of Pab1020 in P. abyssi cells by pull-down and mass spectrometry analyses, and we obtained interesting preliminary results on the interaction, in cellulo, of Pab1020 with partner proteins by co-immunolocalization of proteins by wide-field microscopy (Figure 2).
Figure 2. A. Identification of Pab102 protein parterns (pull-down in cellulo). B. Immunolocalization of Pab1020 protein in P. abyssi cells



