Cell migration and morphogenesis in the early embryo
People
Nicolas David, DR CNRS
Sophie Escot, CR Inserm
Amelie Elouin, PhD student
Yara Hassanein, PhD student
Amandine Ignace, Assistant Engineer
Isabelle Lamarre, Design Engineer
Emilie Menant, Zebrafish husbandry
Topic
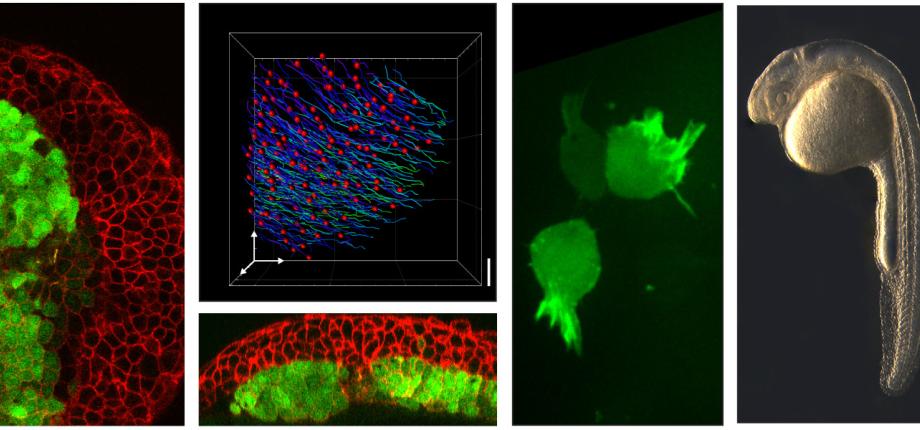
Cell migration is a crucial process for animal development and physiology. Many if not all cell types move at a specific developmental time or in specific situations. Their migration places, shapes, or repairs the tissue of which they are part. In cancer, cell migration is also responsible for metastasis. For the past decades, the cellular and molecular bases of migration have been thoroughly analyzed in vitro. However migration in vivo appears much more complex, migration being largely influenced by the complex cellular environment (neighboring cells, extracellular matrix, chemo-attractants, mechanical constraints…). Cell movements in the organism are thus much less understood. Gastrulation is the first step in development when cells move in the embryo, to set up the future body plan. It is a highly conserved morphogenetic process transforming a seemingly unstructured blastula into a highly organized gastrula-stage embryo composed of the three germ layers ectoderm, mesoderm and endoderm. In addition to its crucial role in embryogenesis, gastrulation is an ideal system to study cell migration since it combines high quality imaging, genetic tools and direct manipulation (transplants, explants, ablations, application/relief of mechanical constraints...), thus allowing for multi-disciplinary approaches. We are using the fish gastrula as a model system to analyze cell migration in vivo, focusing mainly on endoderm and mesoderm migration.
Main methods used:
Live imaging, micro-injections (RNA, morpholinos), cell transplants, transgenic and mutants lines, in situ hybridisation, immuno stainings.
Selected Publications
Inactivating negative regulators of cortical branched actin enhances persistence of single cell migration.
Fokin AI, Boutillon A, James J, Courtois L, Vacher S, Simanov G, Wang Y, Polesskaya A, Bièche I, David NB, Gautreau AM.
Journal of Cell Science (2024)
MiniBAR/KIAA0355 is a dual Rac and Rab effector required for ciliogenesis
Serres M*, Shaughnessy R*, Escot S*, Hammich H, Cuvelier F , Salles A , Rocancourt M, Verdon Q, Gaffuri A-L, Sourigues Y, Malherbe G, Velikovsky L, Chardon F, Tinevez J-Y, Callebaut I, Formstecher E, Houdusse A, David N B, Pylypenko O and Echard A. (*equal contributors)
Chromatically Corrected Multicolor Multiphoton Microscopy
Hugo Blanc, Gabriel Kaddour, Nicolas B David, Willy Supatto, Jean Livet, Emmanuel Beaurepaire, Pierre Mahou.
Collective cell migration due to guidance-by-followers is robust to multiple stimuli
Live 3D imaging and mapping of shear stresses within tissues using incompressible elastic beads.
High-speed polarization-resolved third-harmonic microscopy
Josephine Morizet, Guillaume Ducourthial, Willy Supatto, Arthur Boutillon, Renaud Legouis, Marie-Claire Schanne-Klein, Chiara Stringari, and Emmanuel Beaurepaire
Optica (2019)
Analysis of In Vivo Cell Migration in Mosaic Zebrafish Embryos
Arthur Boutillon, Florence Giger, Nicolas B David
Methods Mol Biol. (2018)
Endodermal germ-layer formation through active actin-driven migration triggered by N-cadherin
Florence Giger, Nicolas B David
PNAS (2017)
Analyzing in vivo cell migration using cell transplantations and time-lapse imaging in zebrafish embryos
Florence Giger, Julien G Dumortier, Nicolas B David
JoVE (2016)
The TORC2 Component, Sin1, Controls Migration of Anterior Mesendoderm during Zebrafish Gastrulation
Julien G Dumortier & Nicolas B David
PlosOne (2015)
Inhibitory signalling to the Arp2/3 complex steers cell migration
Irene Dang, Roman Gorelik [...] Nicolas B David, JV Small, Jan Faix, Laurent Blanchoin, Alexis Gautreau
Nature (2013)
Collective mesendoderm migration relies on an intrinsic directionality signal transmitted through cell contacts
Julien G Dumortier, Sandro Martin, Dirk Meyer, Frederic M Rosa and Nicolas B David
PNAS (2012)
Ongoing collaborations
Alexis Gautreau (BIOC, Ecole Polytechnique, Palaiseau)
Arnaud Echard (Pasteur Institute, Paris)
Lutz Brusch (TU Dresden)
Joseph d'Alessandro (Jacques Monod Institute)
Marcel Tawk (Baulieu Institute, Paris)



